Toxicology application in drug development. In applications of toxicogenomics in safety evaluation and risk assessment eds d.
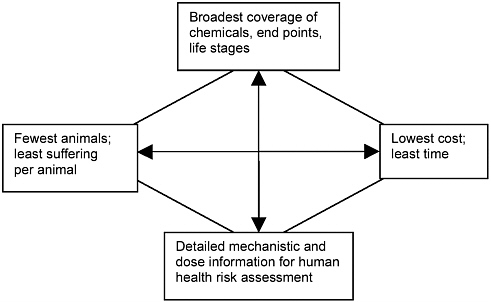 6 New Approaches Toxicity Testing For Assessment Of
6 New Approaches Toxicity Testing For Assessment Of
Applications from us citizens and resident aliens submitted before january 15 will be considered for institutional fellowships.

Toxicology Risk Assessment Practical Considerations Applications Novel Pdf. Outstanding candidates who are us citizens or resident aliens with interest in regulatory science and risk assessment will be considered for funding through niehs funded training grant. Approaches and practical considerations for the analysis of toxicogenomics data. Present risk assessment training to public at toxicology and risk assessment conference to further advance the application of the latest risk assessment.
Toxicology applications of toxicogenomics in safety. Toxicology and risk assessment crc press book. Emerging issues limitations in knowledge and methods considerations of developmental and age sensitivities use of defaults case samples on results in risk assessment and risk management and current and future perspectives.
The relationship between a hazard and potential for exposure defines risk. Methods and applications for risk assessment is an essential reference on the translation of computational toxicology data into information that can be used for more informed risk assessment decision making. Theory and practical applications of systematic review to support regulatory decision making for evidence based risk assessment the use of systematic review sr and evidence based methodologies in toxicology and risk assessment have evolved from theory to practice.
Predictive toxicology describes the study of how toxic effects observed in humans or model systems can be used to predict pathogenesis assess risk and prevent human disease. The participants will have a unique opportunity to learn and apply conventional methodologies detailed considerations and emerging approaches in support of human health risk assessment. Toxicological risk assessments tra we offer toxicological risk assessment tra services for products manufactured and sold in a wide range of industries.
Introductory sections are followed by a series of chapters highlighting practical and systematic applications of toxicogenomics in informing the risk assessment process including the areas of mutagenicity carcinogenicity endocrine toxicity organ specific toxicity population monitoring and ecotoxicology. Overview of risk assessment applications of toxicogenomic technologies to predictive toxicology and risk assessment ncbi bookshelf the objective of chemical risk assessment methodologies is to facilitate both scientific and data informed decision making and also is increasingly expected to provide more accurate predictions of actual risk. Predictive toxicology includes but is not limited to risk assessment the practical facilitation of decision making with scientific information.
Guidance On Information Requirements And Chemical Safety
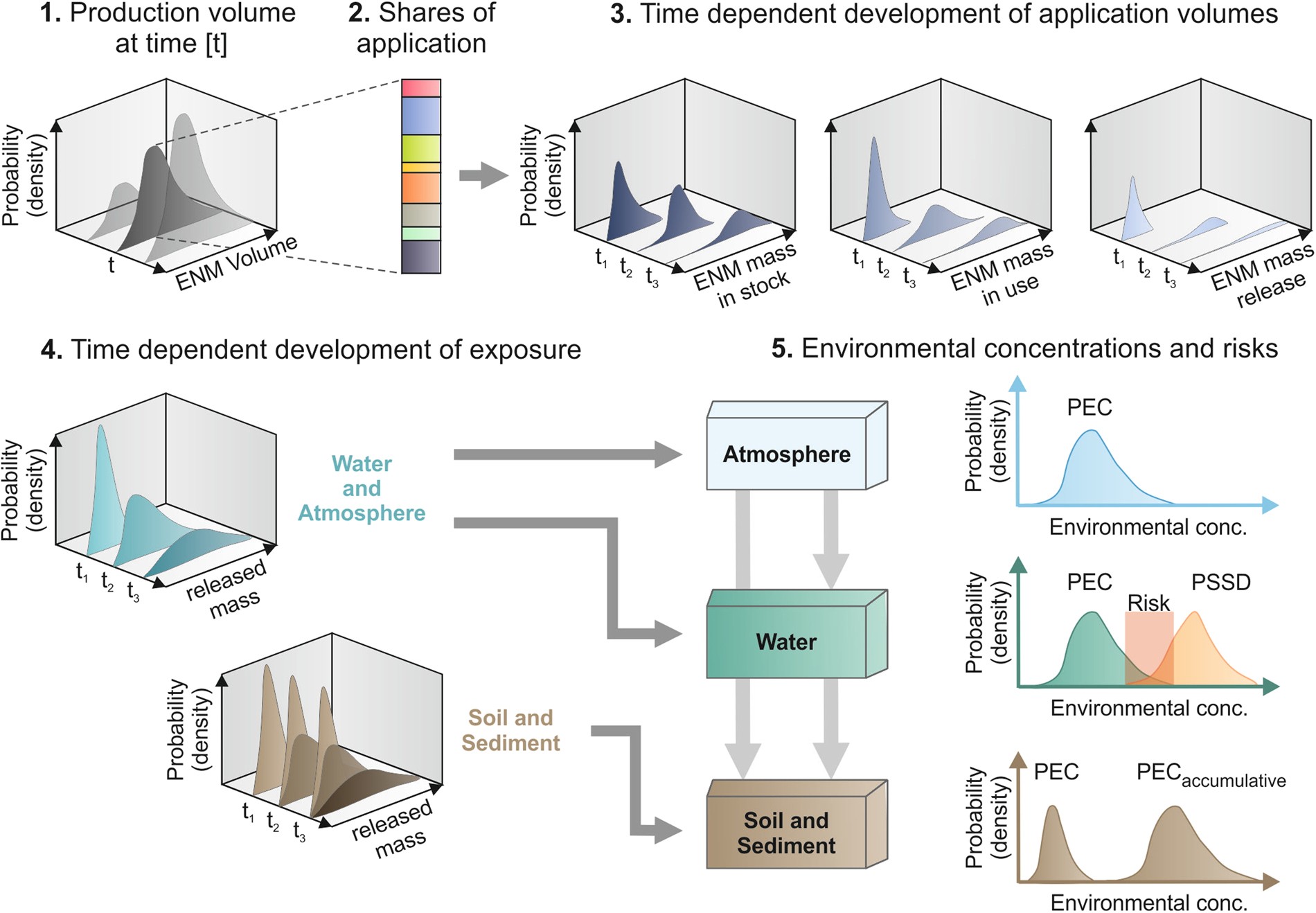 Risks Release And Concentrations Of Engineered Nanomaterial
Risks Release And Concentrations Of Engineered Nanomaterial
 In Silico Toxicology Protocols Sciencedirect
In Silico Toxicology Protocols Sciencedirect
Considerations For Assessing The Risks Of Combined Exposure
Principles And Guidelines For Incorporating Microbiological
 Toxicological Assessment Of Food And Feed Safety Tno
Toxicological Assessment Of Food And Feed Safety Tno
Toxicity And Assessment Of Chemical Mixtures
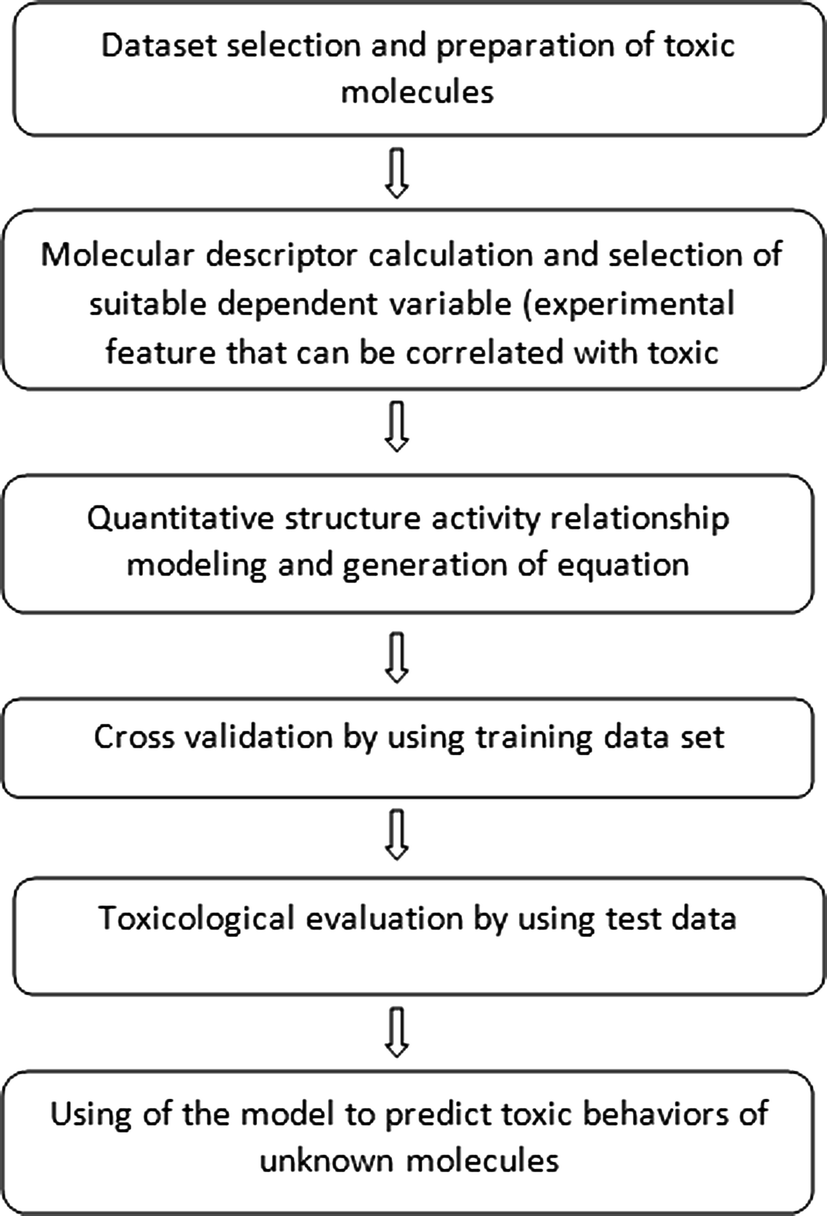 Quantitative Structureactivity Relationship Methods For The
Quantitative Structureactivity Relationship Methods For The
 Risk Management Wikipedia
Risk Management Wikipedia
 A Comprehensive Guide To Toxicology In Nonclinical Drug
A Comprehensive Guide To Toxicology In Nonclinical Drug
 Science Policy Note The Application Of Uncertainty Factors
Science Policy Note The Application Of Uncertainty Factors
 8 Risk Management
8 Risk Management
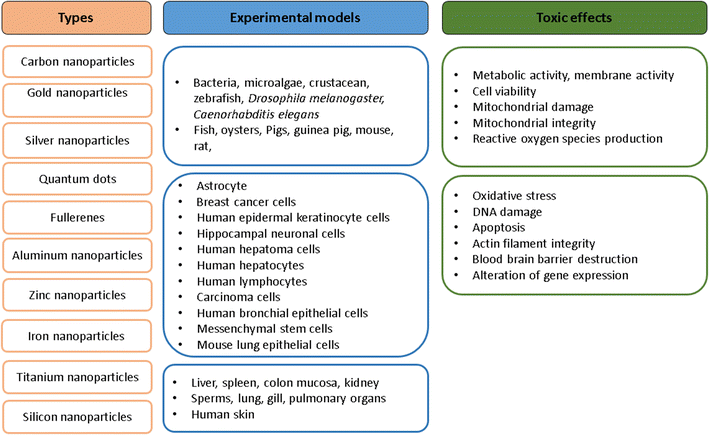 In Vitro And In Vivo Toxicity Assessment Of Nanoparticles
In Vitro And In Vivo Toxicity Assessment Of Nanoparticles
Risk Assessment Of Lmos Training Manual Module 3
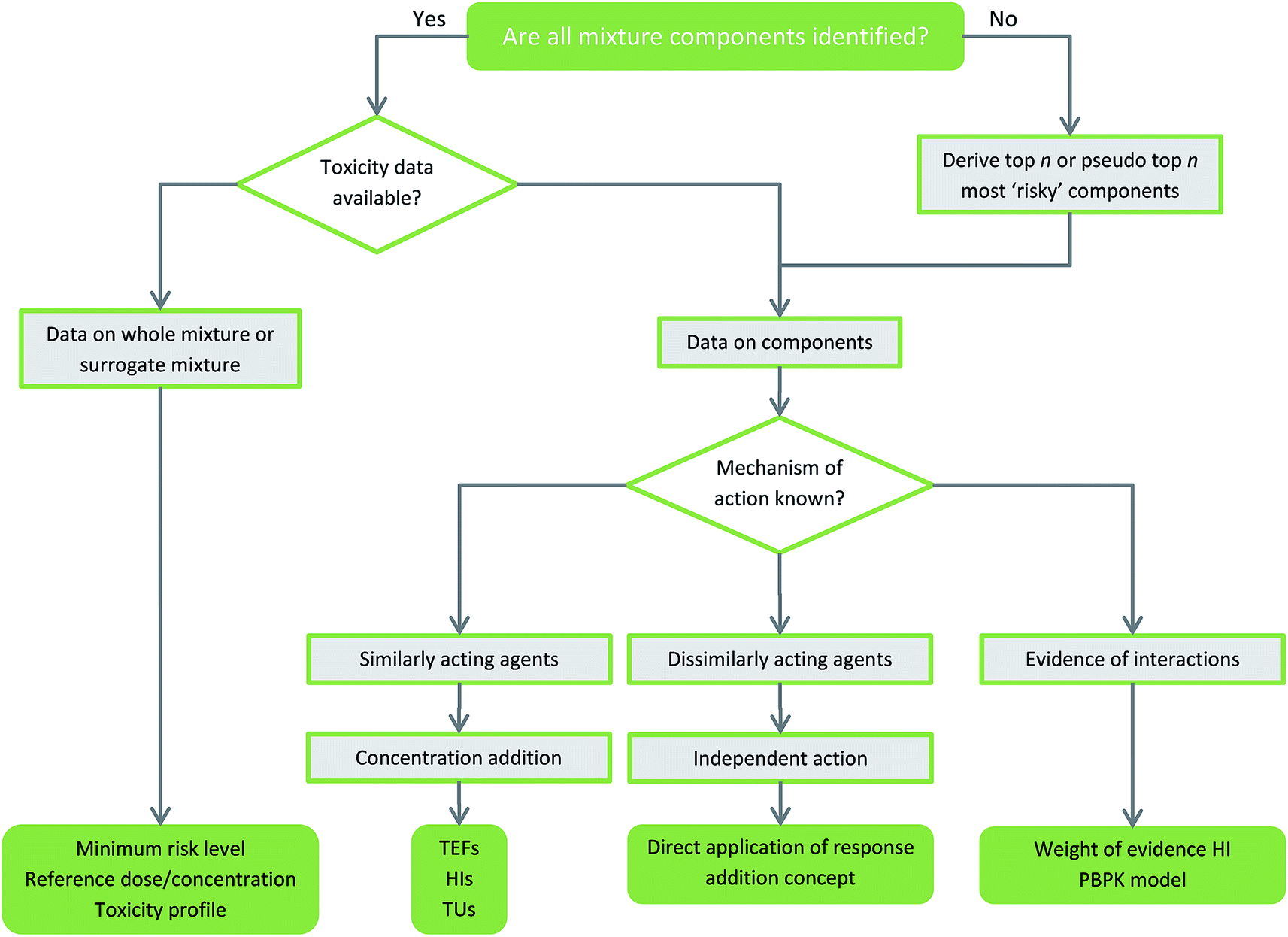 Risk Assessment Of Environmental Mixture Effects Rsc
Risk Assessment Of Environmental Mixture Effects Rsc
